A molecule of ozone consists of three oxygen atoms (O). The chemical formula of ozone is therefore O3.
The name ozone comes from the Greek word ozein, which means to smell. Yes, indeed ozone has a very typical smell. You can easily smell it after a heavy thunderstorm with heavy lightning, or in a small room where a copier or laser printer is working for a long time. The color of this gas with a boiling point of -119.9 °C is light blue. Ozone was already discovered in 1840 by Christian Schönbein.
Ozone is highly reactive, more so than ordinary oxygen gas which consists of only two oxygen atoms. Ozone is a strong disinfectant. As a result, it also has many applications such as disinfecting water (drinking water, spring water, but also sometimes swimming pool water) and bleaching fabrics.
The ozone formed protects us against harmful UV radiation from the sun, because the ultraviolet radiation breaks down the ozone back into oxygen gas and an oxygen atom.
During a heavy thunderstorm with lots of lightning, ozone is formed in the same way as in the stratosphere. Ozone is too reactive, but also too unstable to actually store it. So if you need it, you have to create it on site. You can do this by imitating lightning using electrical discharges in an ozone generator, a device developed by Werner von Siemens.

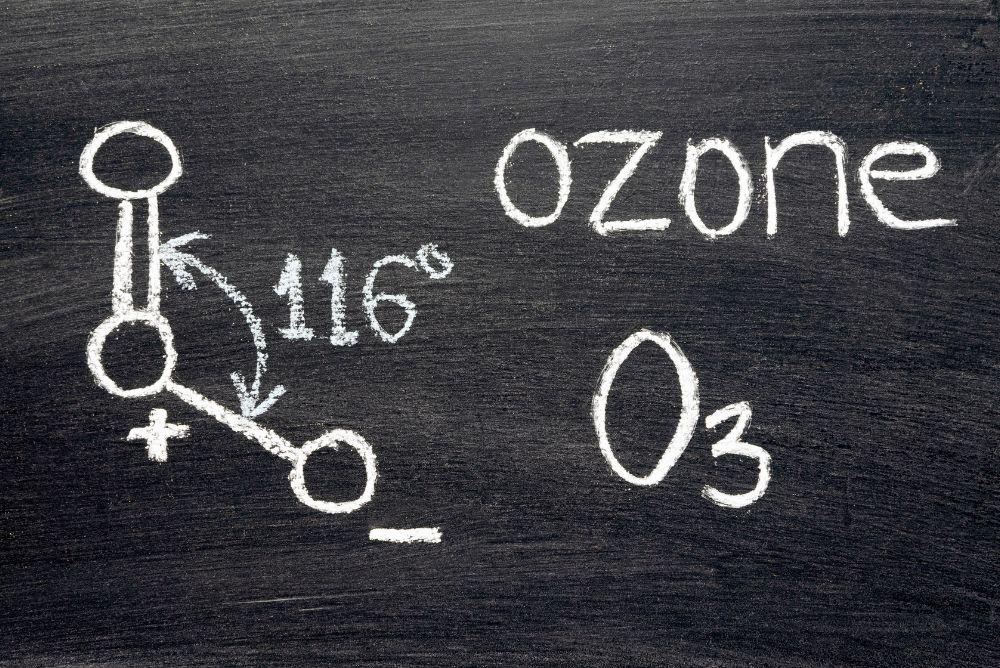
At standard temperature and pressure, ozone is a colorless to pale blue gas with a characteristic pungent odor. In liquid form it is dark blue.
The name comes from the Greek ozein (ὄζειν), which means to smell, smell, smell. This refers to the characteristic odor of the gas.
Ozone has a disinfectant effect and is used, for example, to disinfect drinking water. The advantage over, for example, dichlor is that it does not leave a taste in the water.
Ozone is also used to disinfect well water, swimming water and surfaces that come into contact with food, to remove traces of yeasts from the air (important when food is packed), to clean and bleach fabrics, to deodorize (car) interiors and materials after a fire and when removing unwanted harmful substances (for example herbicides) from water.
In intensive fish farming and aquaculture, ozone is used as a disinfectant and for the breakdown of fish waste products. It is also used as an oxidizer in soil remediations (C-sparge) of organic contaminants (for example BTEX or trichloroethylene).
In organic synthesis, ozone is used in ozonolysis. This reaction is used to oxidatively split alkenes. For example, cyclohexene is oxidized to adipaldehyde: The medical use of ozone still occurs, especially in Cuba to combat cancer.
It finds application as an oxidizing agent, spec. for bleaching organic substances, as a disinfectant (see ozonation) and for air improvement in enclosed spaces.
Sources
Wikipedia
Chem.kuleuven
By Army of Love
Ozonproducten.nl
[email protected]
+31 (0)6 2156 2228
KvK: 82733163
BTW nr: NL862583640B01
Bankrekening: NL98INGB0006985457
